There's some important news you should know if you or someone you care about takes duloxetine, a medicine often used for things like feeling down, feeling worried, or even certain kinds of body aches. The folks at the FDA, which is like the main group that watches over our medicines, have put out a notice about a significant recall. This isn't just a small thing; we're talking about a lot of bottles, over 233,000 of them, because of something called nitrosamine, which, you know, could be a concern for your health in the long run. It's a situation that, in some respects, really makes you think about what's in our everyday medicines.
This big recall, which involves a whole bunch of duloxetine bottles, follows a slightly smaller one that happened back in October 2024. The reason for all this, frankly, has to do with a certain kind of impurity that was found in the medicine, an impurity that has been linked to a higher chance of developing cancer if you're exposed to it over time. So, it's pretty clear why they're taking this so seriously, and why, naturally, people might have questions about their own prescriptions.
The good news, though, is that the FDA has also given some very clear advice for anyone currently using this medicine. They're saying, quite simply, that you should not just stop taking your duloxetine on your own. It's a bit of a tricky spot, because while there's a recall, suddenly stopping a medicine like this can sometimes cause other problems. This is why, in fact, it's always best to talk with your doctor or the person who helps you with your health if you have any worries or need to make a change.
Table of Contents
- What's Going On With Duloxetine?
- The Duloxetine Recall - What the FDA Said
- Why Was This Duloxetine Recall Issued?
- Nitrosamines and the Duloxetine Recall - What's the Big Deal?
- What Does a "Class II" Recall Mean for You?
- The FDA's Advice on the Duloxetine Recall
- What If You're Taking Duloxetine?
- Duloxetine Recall and Your Daily Life
What's Going On With Duloxetine?
You might be hearing some talk about a medicine called duloxetine, and you know, it's causing a bit of a stir. This particular medicine, which has been around for quite a while, approved, as a matter of fact, back in 2004, is suddenly in the spotlight because of some recent actions by the people who look after our medicine safety. It's a situation where, apparently, a large number of bottles are being pulled back from pharmacies and store shelves, which can feel a little unsettling if you happen to take it yourself.
The numbers involved are, quite frankly, rather big. We're talking about more than 233,000 bottles of this antidepressant that have been asked to come back. This action, so you know, isn't something that happens every day, and it's because of something that was found inside the medicine itself. It's a type of substance that, in some ways, just shouldn't be there, especially not in the amounts that were discovered. This is why, typically, when something like this comes up, the authorities act pretty quickly to let everyone know.
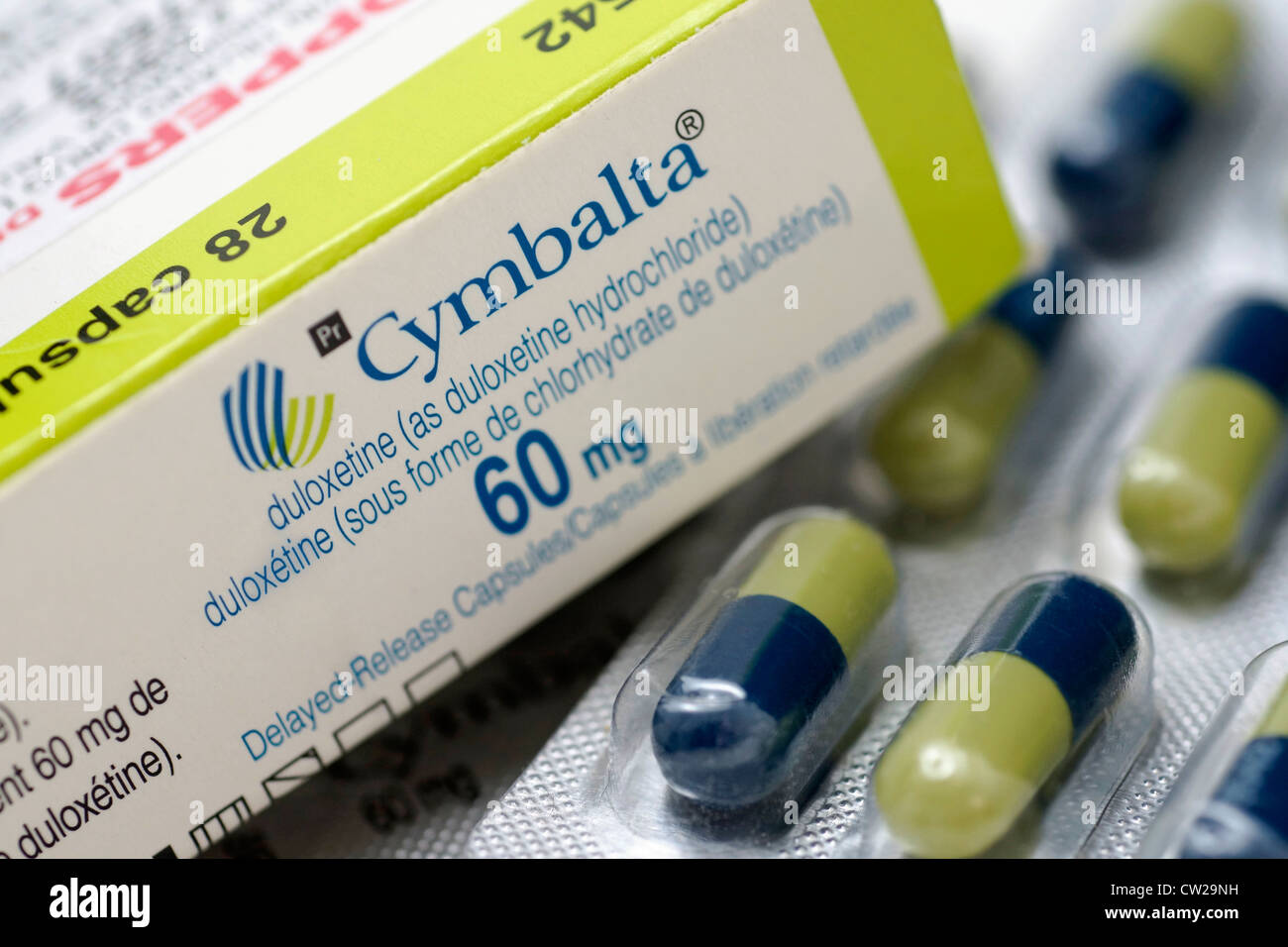
This particular medicine, duloxetine, you see, goes by a few different names, like Cymbalta, which is a brand name many people might recognize. It's used for a whole range of things, from helping people who are dealing with feeling very sad or worried, to easing certain kinds of nerve pain. So, when there's a recall involving a medicine that so many people rely on for various health concerns, it naturally gets a lot of attention, and people want to know the ins and outs of what's happening, and what it means for them, personally.
- Skinny Mindy Kaling
- Nischelle Turner Husband
- Jon Pardi Age
- Tickets For New Years Eve Ball Drop
- Sad Michael Jordan
The Duloxetine Recall - What the FDA Said
The Food and Drug Administration, or FDA as we often call them, has been pretty clear about what's going on with this duloxetine recall. They put out what's known as a "Class II recall" notice, which, in short, means there's a situation where using the product might cause a temporary health problem, or the chance of a serious health problem is not very high. But, you know, they still want people to be aware and take action if needed. This particular notice, actually, was about more than 7,100 bottles of the medicine at first, but then it grew to cover a much larger amount, which is interesting.
This initial action, which involved those 7,107 bottles of duloxetine, was put into motion on December 4th, 2024. The reason given for this first part of the recall was something called "cGMP deviations." Now, "cGMP" stands for current good manufacturing practices, and basically, it's about how medicines are made to make sure they're good quality and safe. So, when there are "deviations," it means the way the medicine was put together didn't quite meet the expected standards. This is why, in a way, the FDA steps in to make sure everything is being done the right way for our safety.
Later on, the situation expanded, and we saw that much larger number of bottles, over 233,000, also being pulled back. This bigger duloxetine recall was, as a matter of fact, voluntarily done by the company that distributes the medicine, a company named Rising. The core issue remained the same: the presence of an unwanted substance, a kind of impurity that just shouldn't be there. It's like, you know, when you're making something and a little bit of something else accidentally gets mixed in, but in this case, that "something else" has potential health implications. The FDA, for instance, keeps a close eye on these things to protect everyone.
Why Was This Duloxetine Recall Issued?
So, you might be wondering, why exactly did this duloxetine recall happen? What was it about this medicine that made the FDA and the manufacturers decide to pull it back? Well, the main thing, honestly, comes down to finding a specific kind of impurity. It's not just any impurity, but one that raises some serious questions about long-term health. This substance, which is the root cause of the problem, was found in amounts that were just too high, higher than what's considered safe for people to take regularly. This is why, naturally, they had to act.
The unwanted guest in these duloxetine bottles is called nitrosamine. Now, that might sound like a very technical word, but what you need to know about nitrosamines is that they are known to be toxic. More importantly, if you're exposed to them over a long period, they may increase the risk of cancer. It's not that taking one dose will cause cancer, but the concern is about continuous exposure. This is why, you know, when they find these things in medicines, it's a big deal, and they need to make sure that people aren't unknowingly taking something that could be harmful later on.
This isn't the first time nitrosamines have been a concern in medicines, either. There have been other recalls of different drugs in the past because of this same type of impurity. So, the FDA has a pretty good system for looking for these things and, in some respects, knows what to do when they find them. The drug in question, this duloxetine, is made by a company called Towa Pharmaceutical Europe, and the distributor for some of these products is Rising. So, it's a bit of a chain, you know, from where it's made to where it ends up in your hands, and everyone along that chain has a part to play in keeping things safe.
Nitrosamines and the Duloxetine Recall - What's the Big Deal?
Let's talk a little more about these nitrosamines, because, frankly, they're the main reason for this whole duloxetine recall. These aren't ingredients that are supposed to be in your medicine; they're more like accidental byproducts or contaminants. They can form in a few different ways, sometimes during the manufacturing process itself, or even from the way certain chemicals react with each other over time. It's like, you know, when you're baking a cake and a tiny bit of something unexpected gets into the batter. But in this case, that "tiny bit" can have some pretty serious implications for your health, particularly over a long period.
The reason why nitrosamines are such a concern is because they are classified as potential human carcinogens. What that really means is that, based on studies and what we understand, being exposed to them might increase your chance of developing cancer. It's not a guarantee, but it's a risk that nobody wants to take, especially not with something you're taking for your health. This is why, in fact, there are very strict limits on how much of these substances, if any, can be present in medicines. If the levels go above that limit, then, as a matter of fact, a recall becomes necessary to protect public well-being.
The FDA, you see, has been very clear that the duloxetine capsules were recalled specifically because the nitrosamine impurity was found "above the limit." This isn't just a slight bit over; it's enough to trigger a significant action. The recall, by the way, affects a number of different batch numbers, which are basically like identification codes for specific groups of medicine made at the same time. These affected batches were sent out to pharmacies all over the country, so it's not just a small, localized issue. It’s a widespread thing, which, you know, means a lot of people could be affected, and that's why they want everyone to be aware.
What Does a "Class II" Recall Mean for You?
When you hear about a "Class II recall," especially concerning something like this duloxetine recall, it's pretty normal to wonder what that actually means for you, the person taking the medicine. It's one of those official terms that can sound a bit scary, but it has a specific meaning that's good to understand. A Class II recall is issued when there's a situation where using the product might cause a temporary health problem, or where the chance of a serious health problem is not very high. It's different from a Class I recall, which is for much more serious, life-threatening situations. So, in a way, it's a middle ground, but still important.
For the duloxetine recall, the Class II designation tells us that while the presence of nitrosamines is a concern because of the potential for increased cancer risk over time, it's not considered an immediate, life-threatening danger. This is why, for instance, the FDA's advice is not to suddenly stop taking your medicine without talking to a doctor first. They're basically saying, "Hey, there's an issue here, and we need to fix it, but don't panic and stop your treatment, because that could cause other problems for your health." It's a balance, you know, between addressing the impurity and making sure people don't experience withdrawal or a worsening of their condition.
The reason for this specific classification, as we mentioned, was due to those "cGMP deviations" and the nitrosamine impurity being above the acceptable limit. It means that the process of making the medicine, or the ingredients themselves, didn't quite meet the strict quality standards that are put in place to keep us safe. So, it's a quality control issue that has health implications. This is why, actually, the FDA takes these things very seriously, and they work with the companies to get these products off the shelves and ensure that future batches are made correctly. It's all about making sure the medicines we take are, essentially, as safe as they can be.
The FDA's Advice on the Duloxetine Recall
One of the most important things the FDA wants everyone to know about this duloxetine recall is a very clear piece of advice: do not just stop taking your medicine on your own. This is really, really important, especially for a drug like duloxetine. You see, this medicine is used to help people with some pretty significant health challenges, like dealing with depression, managing anxiety, and even helping with different kinds of pain, including nerve pain. Suddenly stopping a medicine that helps with these things can, as a matter of fact, lead to some very uncomfortable and even risky withdrawal symptoms or a return of the original problems. So, it's a bit of a balancing act.
The reason for this cautious approach is that the immediate risk from the nitrosamine impurity is considered to be a long-term one, not something that will cause harm right away. The bigger, more immediate risk, in their view, comes from abruptly stopping a medication that your body has become used to. This is why, naturally, they're urging people to talk to their healthcare provider first. Your doctor can help you figure out the best way to move forward, whether that means switching to a different batch, a different brand, or even a different medication altogether. It's about making a plan that keeps you safe and healthy, in a way, through this whole situation.
This advice really highlights the FDA's role in public health. They're not just about finding problems; they're also about guiding people through them in the safest possible way. So, if you're taking duloxetine, and you've heard about this recall, the first step is to get in touch with your doctor or pharmacist. They'll have the most up-to-date information about which specific batches are affected and what the next steps should be for your particular situation. It's like, you know, having a trusted guide when things get a little confusing, and that's what your healthcare team is there for.
What If You're Taking Duloxetine?
If you're currently taking duloxetine, and you've just learned about this recall, it's perfectly normal to feel a bit concerned or to have a lot of questions. This medicine, as we've talked about, is prescribed for a variety of conditions, including helping with mood and nerve issues. So, it's a big part of many people's daily routines. The most important thing to remember, as the FDA has advised, is not to make any sudden changes to your medication schedule. That's really the first and most crucial piece of advice for anyone using this medicine right now, especially with this duloxetine recall in the news.
The recall, you see, specifically involves certain lot numbers of the duloxetine capsules. A "lot number" is basically a unique code that identifies a specific batch of medicine made at one time. For example, one of the affected lot numbers mentioned was 220128. These particular products were distributed to pharmacies across the entire country. So, to find out if your specific bottle of duloxetine is part of the recall, you'd need to check the lot number on your prescription bottle. Your pharmacist can definitely help you with this, and they're usually the best first point of contact for these kinds of questions, honestly.
This situation really emphasizes the importance of keeping open lines of communication with your doctor and pharmacist. They are the ones who can look at your specific prescription, check the lot numbers, and give you personalized advice based on your health needs. They might suggest continuing your current supply while they arrange for a new, unaffected batch, or they might talk about other options. It's a bit like, you know, having a personalized plan when something unexpected comes up, and that's what your healthcare team is there to provide for you.
Duloxetine Recall and Your Daily Life
For people who take duloxetine every day, this duloxetine recall can feel very personal. This medicine is prescribed for a pretty wide range of things, from helping manage feelings of sadness and worry to easing nerve pain, and even for a specific condition in women called stress incontinence, where the brand Yentreve® is sometimes prescribed. So, it touches on many different aspects of people's lives and well-being. It's not just a pill; it's often a key part of managing a chronic condition, which is why, you know, any disruption can be quite unsettling.
We can see from people's experiences that duloxetine can have a big impact. Some people have been on it for many years, like the person who was on 60 mg for six years before switching. Others might just be starting it, perhaps for nerve pain, and noticing new things, like having cold feet, which is interesting. There are also stories about how the medicine is taken, like some people taking it once a day, and others finding it better to split their dose into two, in the morning and late afternoon, especially if symptoms worsen hours after taking it. This shows how, in a way, everyone's experience with medication can be a little different.
Then there are the side effects, which, frankly, can also be a part of the daily experience. One person mentioned having disturbed dreams and nightmares for about 18 months while on duloxetine. These personal stories really highlight that medicines aren't just chemical compounds; they affect real people in real ways. So, when a recall happens, it's not just about a product; it's about potentially affecting someone's routine, their well-being, and how they manage their health every single day. This is why, as a matter of fact, getting clear, human-centered information about something like the duloxetine recall is so important for everyone involved.
- Kail Javi
- Kris Jenner Short Hairstyles
- Monsters Cast Menendez Brothers
- Bone Thugs N Harmon
- Brad Pitt And Angelina Jolie Twins